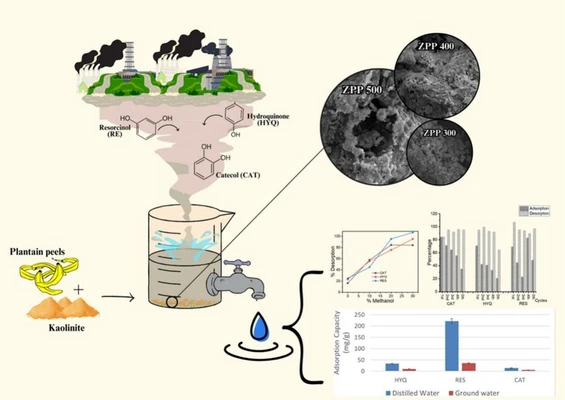
Phenolic pollutants such as hydroquinone (HYQ), resorcinol (RES), and catechol (CAT) pose significant environmental and health risks due to their persistence and toxicity in water systems. This study synthesized sustainable zinc tungstate-based composites through a mechanothermal process using zinc acetate and sodium tungstate in the presence of waste plantain peel and kaolinite clay. The resulting composites (ZPP300, ZPP400, ZPP500) were characterized and evaluated for the adsorption of HYQ, RES, and CAT in both simulated (distilled) and real (groundwater) water systems. Structural analysis confirmed the formation of ZnWO4 and ZnO crystalline phases and surface functionalities suitable for adsorption. Among the composites, ZPP300 exhibited the highest adsorption capacity (up to 250 mg·g−1 for RES). Adsorption kinetics followed the Brouers-Weron-Sotolongo (BWS) model, indicating heterogeneous surface behavior with rapid adsorption (half-lives ranging from 0.12 to 25 min). Response Surface Methodology (RSM) based on Box–Behnken design effectively optimized key variables such as pH, adsorbent dose, ionic strength, and initial concentration. Real groundwater tests confirmed the adsorbent's practical relevance, with catechol showing stable removal efficiency. Regeneration experiments demonstrated good reusability, especially with methanol desorption. The findings highlight the potential of ZPP composites for tackling phenolic pollution in diverse water matrices, providing a green and practical solution for sustainable water treatment technologies.